Saturday, December 12, 2009
Glass and plastic cups
At dinner at a nice but not very nice restaurant the cups could have been either thin light glass or a convincing plastic immitation. To determine which kind they were we tapped the cups with a metal knife. The cups chimed, therefore they were made of glass.
The best guess I have for this is that a glass cup is essentialy one cystal of silicon dioxide whereas a plastic cup is several long chain polymers linked together by strong intermolecular forces. The result is that when force is applied to the glass cup the force translates smoothly through the cup and at the opposite end of the cup the force reverses direction and travels back through the cup. Thus the resonance is the velocity of the comperssion wave through the cup divided by the length. A plastic cup doesn't resonate becuase it's non crystaline structure does not have enough uniformity for the pressure waves to bounce back and forth within it.
- Posted using BlogPress from my iPhone
Tuesday, November 10, 2009
White and Black
A simple idea: paint roofs, roads, and everything else white instead of black. The underlying idea is that the black color absorbs sunlight and converts it to heat energy while white color reflects the light back into space.
Energy Secretary Chu thinks
Does the idea hold up?
The quote from Chu can be misleading. What is the equivalence? The most likely equivalence is that the CO2 emissions from all cars over 11 years will trap some amount of light energy here. That same amount of light energy will be reflected instead of absorbed by painting things white. Also, what roads? All roads everywhere? Or just in the United States?
Two processes will be discussed to help understand this ambitious plan. First the process by which light absorption increases temperature and second the nature of color in terms of absorption and reflection.
Think about a greenhouse in a garden. It's warm. It's warm because ultraviolet and visible light can penetrate the glass and are absorbed by objects inside the greenhouse. Once absorbed, some light energy is converted to heat, making the object warmer, and the rest is emitted as light of a lower wavelength. Light incident as visible and ultraviolet light is converted into infrared light by this process. Infrared light is unable to penetrate glass and so infrared light is trapped inside a greenhouse bouncing from object to object until it is entirely converted to heat energy.
A white object reflects all light and absorbs none. A red object is red because it reflects red light and absorbs other colors of light. A dark object reflects less of the light than it absorbs. A black object reflects no light and absorbs all of it. This is a physical interpretation of light and coupled with the above discussion it is plain to see what significance color has.
Painting surfaces white will reflect light back up into the atmosphere instead of converting it to heat. Much of this light will make it out into space.
The idea is scientifically sound, but practicality issues of course present themselves.
Energy Secretary Chu thinks
"making roads and roofs a paler color would be equivalent to taking all cars worldwide off the road for 11 years."Chu, by the way, won the 1997 Nobel Prize in Physics. Read about it here.
Does the idea hold up?
The quote from Chu can be misleading. What is the equivalence? The most likely equivalence is that the CO2 emissions from all cars over 11 years will trap some amount of light energy here. That same amount of light energy will be reflected instead of absorbed by painting things white. Also, what roads? All roads everywhere? Or just in the United States?
Two processes will be discussed to help understand this ambitious plan. First the process by which light absorption increases temperature and second the nature of color in terms of absorption and reflection.
Think about a greenhouse in a garden. It's warm. It's warm because ultraviolet and visible light can penetrate the glass and are absorbed by objects inside the greenhouse. Once absorbed, some light energy is converted to heat, making the object warmer, and the rest is emitted as light of a lower wavelength. Light incident as visible and ultraviolet light is converted into infrared light by this process. Infrared light is unable to penetrate glass and so infrared light is trapped inside a greenhouse bouncing from object to object until it is entirely converted to heat energy.
A white object reflects all light and absorbs none. A red object is red because it reflects red light and absorbs other colors of light. A dark object reflects less of the light than it absorbs. A black object reflects no light and absorbs all of it. This is a physical interpretation of light and coupled with the above discussion it is plain to see what significance color has.
Painting surfaces white will reflect light back up into the atmosphere instead of converting it to heat. Much of this light will make it out into space.
The idea is scientifically sound, but practicality issues of course present themselves.
Thursday, October 1, 2009
50% chance of rocks, folks.
Short summary: They found an exoplanet (that's a planet orbiting around a different star) that has regular rock precipitation. The day side of the planet is hot enough to vaporize minerals into the atmosphere, and the night side is cool enough to re-form them as mineral rain. Sweet.
http://www.examiner.com/examiner/x-1242-Science-News-Examiner~y2009m10d1-A-planet-that-rocks
http://www.examiner.com/examiner/x-1242-Science-News-Examiner~y2009m10d1-A-planet-that-rocks
Thursday, September 17, 2009
SHArK Abstract/Summary
SHArK (Solar Hydrogen Activity Research Kit) with Pipette Method: Distributed research project examines how electrochemical responsiveness of metal oxides to visible light varies with concentration and droplet size.
The SHArK project is a distributed research project begun at the Univ. of Wyoming. It hopes to find a combination of metal oxides that will absorb visibile light and spilt water with the absorbed energy.
This research explores the potential of Cobalt, Aluminum, and Iron oxides as catalysts for spliting water into O2 and H2 gas using only sunlight. It also explores the effect of varying droplet size and metal nitrate concentration when using the pipette method to prepare test plates for the SHArK project.
A hydrogen-based economy will not be feasible without a more efficient method of producing hydrogen gas. The SHArK project is a distributed research project that engages high school and undergraduate students. In the pipette method metal nitrates in acidic solution are pipetted onto a conducting glass plate and are then baked in an oven at 500 degrees celsius for 24 hours. While in the oven, the metal nitrates oxidize to metal oxides. The plates are then rinsed and placed in a small aquarium-like tank in .1 molar sodium hydroxide solution. An electrode is connected to the plate, a counter electrode is connected to a graphite rod which is submerged in the electrolyte solution. A voltage is applied across these two electrodes. This applied voltage is called the bias. Each plate is usually scanned twice, one with a positive .5 volt bias and once with a negative .5 volt bias. Electronics controlled by a computer measure the current passing through the circut in miliAmps. Electrochemical activity of the metal oxides is examined by analyzing changes in current in the circut. When the detected current increases it is assumed that an n-type metal oxide is donating electrons to the current and thus increasing the current. When the detected current decreases it is assumed that a p-type metal oxide has opened up a hole and electrons have sank into it and thus decreasing the current. A 532 nm (which is actually a frequency-doubled 1064nm CO2 laser IIRC) green laser pointer is used to test the metal oxides for visibile light-induced electrochemistry. The laser is mounted on a LEGO(Registered Trademark) platform and LEGO Mindstorms(R) robotics control two mirrors that reflect the laser beam onto the test plate. The computer software imagines the plate as a grid of 180 collumns and 180 rows for a total of 32,400 individual data points per scan. For each data point, the computer turns on the laser, records the current, turns off the laser, and tells the robotics to move the mirrors to aim the laser at the next point. The robotics move the laser beam like a typewriter; single steps to the right until they reach the end of a row, then a long step back to the left hand edge, and a single step down. The computer ouputs a text file consisting of 32,400 numbers which is then processed using an open source program called imageJ which in turn assigns a color to each value and creates a 180 pixel by 180 pixel image of the scan. Each plate generates two images; one scanned with a positive bias and the other with a negative bias. Because the SHArK project is a distributed research project, it's website is also home to it's results database. Anyone can view the results so far of the project if they create a user account and all researchers can post their results to the main results archive.
The SHArK project is a distributed research project begun at the Univ. of Wyoming. It hopes to find a combination of metal oxides that will absorb visibile light and spilt water with the absorbed energy.
This research explores the potential of Cobalt, Aluminum, and Iron oxides as catalysts for spliting water into O2 and H2 gas using only sunlight. It also explores the effect of varying droplet size and metal nitrate concentration when using the pipette method to prepare test plates for the SHArK project.
A hydrogen-based economy will not be feasible without a more efficient method of producing hydrogen gas. The SHArK project is a distributed research project that engages high school and undergraduate students. In the pipette method metal nitrates in acidic solution are pipetted onto a conducting glass plate and are then baked in an oven at 500 degrees celsius for 24 hours. While in the oven, the metal nitrates oxidize to metal oxides. The plates are then rinsed and placed in a small aquarium-like tank in .1 molar sodium hydroxide solution. An electrode is connected to the plate, a counter electrode is connected to a graphite rod which is submerged in the electrolyte solution. A voltage is applied across these two electrodes. This applied voltage is called the bias. Each plate is usually scanned twice, one with a positive .5 volt bias and once with a negative .5 volt bias. Electronics controlled by a computer measure the current passing through the circut in miliAmps. Electrochemical activity of the metal oxides is examined by analyzing changes in current in the circut. When the detected current increases it is assumed that an n-type metal oxide is donating electrons to the current and thus increasing the current. When the detected current decreases it is assumed that a p-type metal oxide has opened up a hole and electrons have sank into it and thus decreasing the current. A 532 nm (which is actually a frequency-doubled 1064nm CO2 laser IIRC) green laser pointer is used to test the metal oxides for visibile light-induced electrochemistry. The laser is mounted on a LEGO(Registered Trademark) platform and LEGO Mindstorms(R) robotics control two mirrors that reflect the laser beam onto the test plate. The computer software imagines the plate as a grid of 180 collumns and 180 rows for a total of 32,400 individual data points per scan. For each data point, the computer turns on the laser, records the current, turns off the laser, and tells the robotics to move the mirrors to aim the laser at the next point. The robotics move the laser beam like a typewriter; single steps to the right until they reach the end of a row, then a long step back to the left hand edge, and a single step down. The computer ouputs a text file consisting of 32,400 numbers which is then processed using an open source program called imageJ which in turn assigns a color to each value and creates a 180 pixel by 180 pixel image of the scan. Each plate generates two images; one scanned with a positive bias and the other with a negative bias. Because the SHArK project is a distributed research project, it's website is also home to it's results database. Anyone can view the results so far of the project if they create a user account and all researchers can post their results to the main results archive.
Pentacene
Pentacene might be aromatic. It is Five fused benzene rings. And IBM took a sweet picture of it using(I guess) the pauli exclusion principle quantum force. Which doesn't jive with my science learnings. But I do not know all of quantum mechanics, and as soon as I learn how this here buisness was done I will blog about it at length. Until then, it gets a very high "awesome" rating from me. Go IBM. Yay especially for companies funding pure research!
Here is the link.
Pentacene Pics!
Here is the link.
Pentacene Pics!
Elementeo is a sweet Card Game!
A new board game called elementeo is available online for 35 dollars. I expect it to be quite fun for any junior scientists out there. Or some older scientists looking to play like junior scientists. Or some older scientists who are worried junior is reading to much history and not enough science. Or for some older scientists who have a non-scientist significant other and want a fun way to bring them up to speed on science. Party on yall. I want to play this game so if any of you readers get it, please send me some pics and a review of the gameplay.
Tuesday, September 8, 2009
New SHArK Data!

This scan was performed on an Aluminum-Cobalt-Iron plate. We called the plate P2, because it was the second plate to be prepared by the pipette method. The lower right hot-spot is electrochemical activity from cupric oxide. The two hot spots on the left are cobalt oxide. The two spots at the top right are copper. The water line caused the distortion at the top of the scan. The spots are in different places because light travels through air at a different angle than it travels through water. See Snells law. Email me for this SHArK project data sheet.
Monday, September 7, 2009
Nouveau
Nouveau is an open source project. It aims to get 3D acceleration from nVidia cards. nVidia is a company that makes graphics cards. nVidia writes software that runs on the cards but copywrites this software. Open source software is not copywrited and is written by groups such as nouveau. If nouveau is successful, I will be able to use my Microsoft Xbox to push high-definition video to my television. The Xbox has an nVidia card and tv-out, but without hardware acceleration, it will not be able to put high-def on the screen. An Xbox is ideal for use as a MythTV frontend. MythTV is basically TiVo.
nouveau.freedesktop.org/wiki
nouveau.freedesktop.org/wiki
Friday, September 4, 2009
SHArK project results are in!

The SHArK project produced results today. But don't get all excited. This picture is exactly what the scanning station recorded. Something has gone wrong, we are checking for errors in the scanning station. This was a plate with combinations of Cobalt, Aluminum, Copper, and Iron deposited by the pipette method. Each deposition was duplicated somewhere else on the slide. Each droplet measured precisely 5.0 microliters. Each droplet had a .35 molar concentration of metal nitrate salt, .015 molar concentration of nitric acid, and a .6 molar concentration of ammonium nitrate. The plate was prepared 8/31/09 and fired for approximately 24 hrs. Finally it was scanned 9/2/09. It was placed in a 0.1 molar sodium hydroxide solution and a .5 volt positive bias was applied to it.
This plate is plate P1 because it is the first plate prepared by the pipette method.
The results are unsatisfactory.
I can be emailed at krum.spencer@gmail.com if anyone wants the text output before I put it up on the internet somewhere.
Tuesday, August 25, 2009
SHArK

I am now working on a research project called the SHArK project at Portland State University.
SHArK stands for Student Hydrogen Activity Research Kit. The "R" is small because the logo is four chemical abbreviations; S for sulfur, H for Hydrogen, Ar for Argon, and K for potassium.
The goal of the project is to find a metal catalyst that will produce hydrogen and oxygen gas from liquid water using sunlight energy.
Hopefully a combination of metal oxides in relative concentrations will exhibit this property.
Check out The SHArK Project.
Saturday, March 28, 2009
Not quite science... Nerdy enough though...

I was playing chess against the computer using xboard today. I was playing as white, partially because I love to play 1. e4 and partially because I haven't bothered figuring out how to play against the computer as black. I got into the above position, and I lost. I've tried a few different permutations but always lose as white. Can anyone out there in cyberspace win as white?
Friday, March 27, 2009
Colloids
Milk is a colloid. A homogeneous mixture of fluids that are not miscible. When a ball made up of the molecules of the fluid that appears in lesser proportions forms, such that each molecule is oriented in the same direction away from a central point at the center of the ball, the sphere that is formed is called a micelle. Milk is not a suspension. A suspension has nonuniform distribution of particles in liquid, and will eventually form an emulsion. A good example of a suspension is a snow globe after it has been shaken vigorously.
Saturday, March 14, 2009
Spencer Krum on Really Truly Excellent Pictures
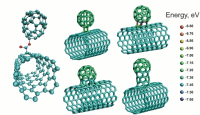
Hello World.
I have been neglecting you, trust that I have not forgotten this project. More math puzzles should be forthcoming. And a major plastics post is in the works, including my confession about something I misled you all on. But for now, I ask you to view the following Image on wikipedia.
It's a nanobud. I know, right? you're thinking this: "I do not know what that is. But look at it! Its a carbon nanotube with a ball sticking out the top! How fuckin' cool is that?! Thats totally cool science."
I wish I could tell you what that nanobud does. I do not know. The wikipedia article touches on "fullerene chemistry" which refers to the molecule family named after Buckminister Fuller. To understand what a Fullerene is, I have to arm you with some knowledge about carbon.
Carbon is the 12th element on the periodic table of the elements. Carbon can bond to other atoms to form molecules like cellulose, which is the "structural component of the primary cell wall in green plants." Notice that the chemical formula for cellulose is C6 H10 05, meaning that for every six carbon atoms there will also be ten hydrogen atoms and five oxygen atoms. Also notice the lower case 'n' subscript, this means that cellulose is a long molecule composed of n-many blocks of C6H10O5 linked together.
Trees stand up because of the rigid structure of long chains of cellulose. When a tree dies and falls down, it dries out, we're all fairly familiar with this process. Cellulose has two hydrogen molecules for each oxygen molecule, so after a long evaporation period, a tree loses ten hydrogen molecules and five oxygen molecules per cellulose monomer, and what is left behind is six carbon atoms in a molecule. If you follow the carbon link above you can see carbons laid out in sheets of hexagons connected to each other. That is graphite. Graphite is what is left behind when a tree is allowed to decompose for a very long time. Fossil fuels, which are carbon and hydrogen chains, are trees and other green plants that have not been decomposing for nearly as long. When graphite sinks below the surface of the earth, and approaches very high temperatures and pressures graphite will rearrange to form diamond. In diamond, each carbon connects to four other carbon atoms in a tetrahedral structure. Tetrahedrons are solid 3-dimensional shapes with four faces all the same, the triangular pyramid is a tetrahedron, so long as no one point is different from the others. In Graphite, by contrast, each carbon connects to three other carbon atoms in a triangular structure. Notice that triangles are 2 dimensional and diamonds three dimensional, and that there are no strong connections between the parallel sheets of graphite in the picture. That is why graphite pencil lead can slide off a pencil tip and onto paper.
The differences between diamond and graphite are allotropic differences. Allotropes are molecules that are composed of the same single species of atom, but have different physical and chemical properties because the atoms are connected to each other in different ways. The carbon atoms in diamond make four bonds to other carbons while the carbon atoms in graphite make only three bonds.
The molecule in the middle of your carbon page above, the buckyball, is another allotrope of carbon. Its atoms are arranged differently, as you can plainly see, and it is very unique because of it's closed ball structure.
The buckyball occurs naturally in soot, though that had very little to do with its discovery in 1985. It was named for Buckminster Fuller because he was the originator of the geodesic dome, the atomic structure that the buckyball molecule is believed to have.
Buckyballs are Fullerenes. Similar to buckyballs are carbon nanotubes, the synthesis of carbon nanotubes is a kind of fullerene chemistry.
To date, fullerene chemistry has been something of a let down. There are some truly promising things about nanotubes and buckyballs, but there have not yet been any consumer products or lifestyle changes because of nanotube technology. When buckyballs were first discovered and studied, scientists thought they could be used as the smallest ball bearings imaginable, and that all machines equipped with buckball bearings would experience enormously tiny friction. They were wrong, buckyballs cannot be used in that way.
One of my favorite chemistry professors had a very funny line about buckyballs: "The only thing that buckballs have been useful for is generating doctoral dissertations."
Stay classy, world.
GNU See above.
Friday, March 6, 2009
Math Problem 3
A farmer plants A acres of wheat one year. Each year thereafter, he harvests (removes) 1/4 of the planted acreage and then plants 1500 more acres. The number of acres of wheat planted approaches what number?
A) 3000
B) 4000
C) 5000
D) 6000
E) It depends on the value of A.
A) 3000
B) 4000
C) 5000
D) 6000
E) It depends on the value of A.
Thursday, March 5, 2009
Wednesday, March 4, 2009
Math Problem 2

AMATYC Student Mathematics League
For how many integer values of K do the graphs of X + Y = K and XY = K NOT intersect?
Tuesday, March 3, 2009
Math Problem 1

AMATYC Student Mathematics League
One can of frozen juice concentrate, when mixed with 4 1/3 cans of water, makes 2 quarts (64 oz) of juice. Assuming no volume is gained or lost by mixing, how many oz does a can hold?
Thursday, February 26, 2009

Can we reuse plastic bottles?
I believe that we can use water bottles, and really everything else we now call trash, as raw materials. What we need is a little ingenuity to see how precisely to get from raw materials to finished product.
My father and I have been bouncing ideas back and forth for months about how we could reuse plastic bottles to build small houses or shelters. The homeless in America, as well as others around the world, could take bottles and other materials out of landfills and use them to build shelters.
This kid has exactly the same idea. And while that dome isn't made from reused products, in principle other domes could be made by reusing trash more like this guy.
The last chapter (chapter 26) of my Organic Chemistry book is on Synthetic Polymers. The plastic bottles are made of polyethylene. "At low temperatures, long-chain polymers are glasses." If we subject them to stresses, they will tend to be rigid and inflexible, shock force will cause fractures.
The material is not malleable nor is it ductile at room temperatures. When the material is heated beyond its glass transition temperature and into its thermoplastic phase it does achieve malleability. If the material is heated further to its crystalline melting temperature then it achieves malleability and ductility.
"A determination of the softening point for materials such as polyethylene, which have no definite melting point. It is taken as the temperature at which the specimen is penetrated to a depth of 1 mm by a flat-ended needle with a 1 sq. mm circular or square cross-section, under a 1000-gm load. Also known as Vicat softening temperature.
Definition Copyright ©1989 CRC Press LLC. All rights reserved."
The plastic bottles are a kind of Low-Density polyethylene. Low density corresponds to less conformity to a crystalline structure and so is less rigid. There is a Google Book that reports on the physical properties of various chemicals, and determines the vicat softening temperature for LDPE (Low-Density PolyEthylene) to be between 80 and 98 degrees Celsius.
But what is important is that malleability, which is "being capable of being pounded into sheets", does not occur at the vicat softening temperature. The result is that one bottle can be deformed and manipulated, but not joined to another bottle on a chemical level the way a metal can be.
For these reasons, I believe the best results for reusing plastic bottles do not come from chemistry, but from engineering cuts and folds on the bottles. I think the best structure that can be built by reusing plastic bottles will not attempt to melt down or smash or chemistry the bottles. It will come from the engineer who has the most experience with oragami.
Subscribe to:
Posts (Atom)